GOST R 52407-2005
Group H09
NATIONAL STANDARD OF THE RUSSIAN FEDERATION
DRINKING WATER
Methods for determining stiffness
drinking water. Methods of hardness determination
OKS 13.060.20
OKP 01 3100
Introduction date 2007-01-01
Foreword
Goals and principles of standardization in Russian Federation installed federal law dated December 27, 2002 N 184-FZ "On technical regulation", and the rules for the application of national standards of the Russian Federation - GOST R 1.0-2004 * "Standardization in the Russian Federation. Basic provisions"
________________
* The document is not valid on the territory of the Russian Federation. GOST R 1.0-2012 is in force. - Database manufacturer's note.
About the standard
1 DEVELOPED AND INTRODUCED by the Technical Committee for Standardization TK 343 "Water Quality" (GUP "Center for Research and Water Control", FSUE "VSEGINGEO", FSUE "VNIIstandart", OOO "Protektor")
2 APPROVED AND INTRODUCED BY Order federal agency on technical regulation and metrology of December 20, 2005 N 317-st
3 This standard takes into account the main regulatory provisions of the following international standards ISO:
ISO 6059-1984 * "Water quality - Determination of the sum of calcium and magnesium - EDTA titrimetric method" (Section 4 of this standard);
________________
* Access to international and foreign documents mentioned hereinafter in the text can be obtained by clicking on the link to the site http://shop.cntd.ru. - Database manufacturer's note.
ISO 7980-1986 "Water quality - Determination of calcium and magnesium - Atomic absorption spectrometric method" (clause 5.1 of this standard);
ISO 11885-1996 "Water quality - Determination of 33 elements by inductively coupled plasma atomic emission spectroscopy" (clause 5.2 of this standard)
4 INTRODUCED FOR THE FIRST TIME
5 REVISION. July 2007
Information about changes to this standard is published in the annually published information index "National Standards", and the text of changes and amendments - in the monthly published information indexes "National Standards". In case of revision (replacement) or cancellation of this standard, a corresponding notice will be published in the monthly published information index "National Standards". Relevant information, notification and texts are also placed in information system common use- on the official website of the national body of the Russian Federation for standardization on the Internet
Introduction
Introduction
Water hardness is one of the main indicators characterizing the use of water in various industries.
Water hardness is a set of properties determined by the content of alkaline earth elements in it, mainly calcium and magnesium ions.
Depending on the pH and alkalinity of the water, hardness above 10°F can cause sludge to form in the water distribution system and scale when heated. Water with a hardness of less than 5°F can be corrosive to water pipes. The hardness of water can also affect the applicability for human consumption in terms of its taste properties.
In the complexometric (titrimetric) determination of hardness, aluminum, cadmium, lead, iron, cobalt, copper, manganese, tin and zinc ions affect the establishment of the equivalent point and interfere with the determination. Orthophosphate and carbonate ions can precipitate calcium under titration conditions. Certain organic substances may also interfere with the determination. If the interfering influence cannot be eliminated, it is recommended to determine the hardness using atomic spectrometry methods.
This standard provides for the use of various methods for determining water hardness, taking into account bringing the quantitative characteristics of water hardness (hardness units) in accordance with GOST R 52029.
1 area of use
This standard applies to drinking and natural waters, including drinking water sources, and establishes the following methods for determining water hardness:
- complexometric method (method A);
- methods of atomic spectrometry (methods B and C).
Method B according to 5.1 is used to determine mass concentration calcium and magnesium ions.
Method B of 5.2 is an arbiter of other stiffness methods.
2 Normative references
This standard uses normative references to the following standards:
GOST 8.315-97 State system ensuring the uniformity of measurements. Standard samples of the composition and properties of substances and materials. Key points
GOST 1770-74 Measuring laboratory glassware. Cylinders, beakers, flasks, test tubes. General specifications
GOST 2053-77 Sodium sulfide 9-water. Specifications
GOST 3118-77 Hydrochloric acid. Specifications
GOST 3760-79 Water ammonia. Specifications
GOST 3773-72 Ammonium chloride. Specifications
GOST 4233-77 Sodium chloride. Specifications
GOST 4328-77 Reagents. sodium hydroxide. Specifications
GOST 4461-77 Nitric acid. Specifications
GOST 5456-79 Hydroxylamine hydrochloride. Specifications
GOST 5457-75 Technical dissolved and gaseous acetylene. Specifications
GOST 6709-72 Distilled water. Specifications
GOST 10652-73 Disodium salt of ethylenediamine-N,N,N",N"-tetraacetic acid 2-aqueous (trilon B). Specifications
GOST 17433-80 Industrial purity. Compressed air. Pollution classes
GOST 23950-88 Drinking water. Method for determining the mass concentration of strontium
GOST 24104-2001* Laboratory balances. Are common technical requirements
________________
* The document is not valid on the territory of the Russian Federation. GOST R 53228-2008 is valid, hereinafter in the text. - Database manufacturer's note.
GOST 25336-82 Laboratory glassware and equipment. Types, basic parameters and dimensions
GOST 29169-91 (ISO 648-77) Laboratory glassware. Pipettes with one mark
GOST 29227-91 (ISO 835-1-81) Laboratory glassware. Pipettes graduated. Part 1. General requirements
GOST 29251-91 (ISO 385-1-84) Laboratory glassware. Burettes. Part 1. General requirements
GOST R ISO 5725-6-2002 Accuracy (correctness and precision) of measurement methods and results. Part 6. Using precision values in practice
GOST R ISO/IEC 17025-2006* General requirements for the competence of testing and calibration laboratories
________________
* The document is not valid on the territory of the Russian Federation. GOST ISO/IEC 17025-2009 is valid, hereinafter in the text. - Database manufacturer's note.
GOST R 51309-99 Drinking water. Determination of element content by atomic spectrometry
GOST R 51592-2000 Water. General Sampling Requirements
GOST R 51593-2000 Drinking water. Sample selection
GOST R 51652-2000 Rectified ethyl alcohol from food raw materials. Specifications
GOST R 52029-2003 Water. Unit of stiffness
Note - When using this standard, it is advisable to check the validity of reference standards in the public information system - on the official website of the national body of the Russian Federation for standardization on the Internet or according to the annually published information index "National Standards", which is published as of January 1 of the current year, and according to the corresponding monthly published information indexes published in the current year. If reference document replaced (modified), then when using this standard, you should be guided by the replaced (modified) document. If the referenced document is canceled without replacement, the provision in which the link to it is given applies to the extent that this link is not affected.
3 Sampling
General requirements for sampling - according to GOST R 51592 and GOST R 51593.
A sample is taken with a volume of at least 400 ml for analysis according to method A and at least 200 ml for analysis according to methods B and C in a container made of polymer materials or glass.
The shelf life of a water sample is no more than 24 hours.
To increase the shelf life of the sample and to prevent the precipitation of calcium carbonates from the water (which is typical for groundwater or bottled water), the sample is acidified with acid to pH<2. При определении жесткости по методу А подкисление проводят соляной кислотой, по методу Б - соляной или азотной кислотой, при использовании метода В - азотной кислотой. Контроль рН проводят по универсальной индикаторной бумаге или с использованием рН-метра. Срок хранения подкисленной пробы воды - не более одного месяца.
4 Complexometric method (method A)
4.1 The method is based on the formation of complex compounds of Trilon B with ions of alkaline earth elements. The determination is carried out by titrating the sample with a solution of Trilon B at pH=10 in the presence of an indicator. The lowest determined water hardness is 0.1 °J.
If the test sample has been acidified for preservation or the sample is acidic, add sodium hydroxide solution (see 4.3.8) to an aliquot of the sample until pH=6-7. If the water sample has a strongly alkaline environment, then a hydrochloric acid solution (see 4.3.7) is added to an aliquot of the sample until pH=6-7. pH control is carried out using universal indicator paper or using a pH meter. To remove carbonate and bicarbonate ions from water (which is typical for underground or bottled waters), after adding hydrochloric acid to an aliquot of a sample of a solution of hydrochloric acid to pH = 6-7, it is boiled or blown with air or any inert gas for at least five minutes to remove carbon dioxide. The alkaline reaction of water can serve as a criterion for the presence of a significant amount of carbonates in water.
The presence in the water of more than 10 mg/dm of iron ions, more than 0.05 mg/dm of each of the ions of copper, cadmium, cobalt, lead, more than 0.1 mg/dm of each of the ions of manganese (II), aluminum, zinc, cobalt, nickel, tin, as well as a color of more than 200 ° and increased turbidity cause a fuzzy color change at the equivalence point during titration and lead to an overestimation stiffness results. Orthophosphate and carbonate ions can precipitate calcium under titration conditions at pH=10.
To reduce the effect of zinc contained in water up to 200 mg/dm3, aluminum, cadmium, lead up to 20 mg/dm3, iron up to 5 mg/dm3, manganese, cobalt, copper, nickel up to 1 mg/dm3, 2 ml of sodium sulfide solution is added to the sample aliquot before the indicator is introduced (see 4.3.6); to reduce the effect of manganese to 1 mg/dm, iron, aluminum to 20 mg/dm, copper to 0.3 mg/dm add 5 to 10 drops of hydroxylamine hydrochloride solution (see 4.3.5). Turbidity (suspended matter) of the sample is removed by filtration through membrane filters with a pore diameter of 0.45 µm or paper deashed "blue tape" filters. The influence of color and other factors is eliminated by diluting the sample during the analysis according to 4.5, if the determined value of water hardness allows this.
NOTE Filtration of the sample may lead to an underestimation of the hardness of the water, especially alkaline water.
If interfering influences cannot be eliminated, then the determination of stiffness is carried out by methods of atomic spectrometry.
4.2 Measuring instruments, auxiliary equipment, reagents, materials
The state standard sample (hereinafter referred to as GSO) according to GOST 8.315 of the composition of hardness (total hardness) of water with a relative error of the certified value at a confidence level of 0.95 is not more than 1.5%.
Laboratory scales with a division value of not more than 0.01 g and a maximum weighing limit of 210 g according to GOST 24104.
pH meter of any type.
Volumetric flasks of the 2nd accuracy class according to GOST 1770.
Graduated pipettes of the 2nd accuracy class according to GOST 29227 or pipettes with one mark of the 2nd accuracy class according to GOST 29169.
Burettes of the 2nd class of accuracy with a capacity of 25 cm3 and (or) 10 cm3 according to GOST 29251.
Measuring cylinders (beakers) according to GOST 1770.
Flat-bottomed or conical flasks according to GOST 25336.
Dropper 2-50 XC according to GOST 25336.
Funnels laboratory in accordance with GOST 25336.
Glasses chemical heat-resistant in accordance with GOST 25336.
Device for filtering samples using membrane filters.
Membrane filters with pores 0.45 µm in diameter or paper ashless filters "blue tape".
Cabinet drying laboratory, maintaining the temperature (80±5) °C.
Paper universal indicator for pH control.
Distilled water according to GOST 6709 and (or) bidistilled water (distilled water, re-distilled in glass containers).
GSO of the composition of Trilon B with a mass fraction of 2-aqueous disodium salt of ethylenediamine-N,N,N",N "-tetraacetic acid, not less than 99.5% or standard titer (fixanal) of Trilon B or Trilon B (ethylenediamine-N,N,N",N "-tetraacetic acid disodium salt 2-aqueous) according to GOST 10652, analytical grade. or h.h.
GRM of the composition of an aqueous solution of magnesium ions with a relative error of the certified value at a confidence level of 0.95 not more than 1.0% or a standard titer (fixanal) of magnesium sulfate (sulphate).
Standard titer (fixanal) of hydrochloric acid or nitric acid with a molar concentration of 0.1 mol/dm.
The indicator is eriochrome black T (chromogenic black ET) or chrome dark blue acidic (acid chromic blue T).
Ammonium chloride according to GOST 3773, analytical grade
Water ammonia according to GOST 3760 (25%), chemically pure
Hydrochloric acid according to GOST 3118, chemically pure, or nitric acid according to GOST 4461, chemically pure.
Sodium hydroxide according to GOST 4328, chemically pure
Sodium chloride according to GOST 4233, chemically pure
Sodium sulfide according to GOST 2053, analytical grade
Hydroxylamine hydrochloride according to GOST 5456, analytical grade. or h.h.
Rectified ethyl alcohol according to GOST R 51652.
Note - It is allowed to use other reagents with technical and metrological characteristics not worse than those indicated.
4.3 Preparation of solutions and indicators
4.3.1 Trilon B solution, 25 mmol/l molar concentration
Trilon B is dried at 80 °C for two hours, 9.31 g is weighed, placed in a volumetric flask with a capacity of 1000 cm 3, dissolved in bidistilled water warm from 40 °C to 60 °C, and after cooling the solution to room temperature, bring to the mark with bidistilled water. The correction factor for the concentration of the Trilon B solution (see 4.4) prepared from the sample is set using the magnesium sulfate solution (see 4.3.2). A solution from GSO composition of Trilon B or a standard titer (fixanal) of Trilon B is prepared in accordance with the instructions for use, diluting it to the required concentration. Trilon B solution is usable for six months. It is recommended to check the value of the correction factor at least once a month.
4.3.2 Solution of magnesium ions with a molar concentration of 25 mmol/dm
The solution is prepared from the GSO of the composition of an aqueous solution of magnesium ions or a standard titer (fixanal) of magnesium sulfate (sulphate) in accordance with the instructions for its use, if necessary, diluting to the required concentration.
Note - If in the used standard titers (fixed channels) or GSO of the composition of aqueous solutions, the concentration of a substance is expressed in normality (n), mg / dm, g / m, etc., it is necessary to recalculate the concentration of the substance in mol / dm.
4.3.3 Buffer solution pH=(10±0.1)
To prepare a 500 ml buffer solution, 10 g of ammonium chloride is placed in a 500 ml volumetric flask, 100 ml of bidistilled water is added to dissolve it and 50 ml of 25% aqueous ammonia, mixed thoroughly and brought to the mark with bidistilled water. The buffer solution is usable for two months when stored in a tightly closed container made of polymeric material. It is recommended to periodically check its pH using a pH meter before using the buffer solution. If the pH value has changed by more than 0.2 pH units, then prepare a new buffer solution.
4.3.4 Indicators
4.3.4.1 Indicator solution
To prepare 100 ml of the indicator solution, place 0.5 g of the indicator eriochrome black T into a beaker with a capacity of at least 100 ml, add 20 ml of the buffer solution, mix thoroughly and add 80 ml of ethyl alcohol. The solution is suitable for use within ten days when stored in a dark glass container.
Instead of the eriochrome black T indicator, it is allowed to use the chrome dark blue acid indicator, the solution of which is prepared in a similar way. The shelf life of this solution is no more than three months.
4.3.4.2 Indicator dry mix
The dry mixture of the indicator is prepared in the following sequence: 0.25 g of eriochrome black T is mixed with 50 g of sodium chloride in a porcelain mortar and thoroughly ground. The mixture is usable for one year when stored in a dark glass container.
4.3.5 Hydroxylamine hydrochloride solution
To prepare 100 ml of a solution, 1 g of hydroxylamine hydrochloride (NHOH·HCI) is dissolved in 100 ml of bidistilled water. The solution is suitable for use within two months.
4.3.6 Sodium sulfide solution
To prepare 100 ml of a solution, 5 g of sodium sulfide NaS 9HO or 3.5 g of NaS 5HO are dissolved in 100 ml of bidistilled water. The solution is prepared on the day of the determinations.
4.3.7 Hydrochloric acid solution, molar concentration 0.1 mol/l
In a volumetric flask with a capacity of 1000 ml, half-filled with bidistilled water, pour 8 ml of hydrochloric acid and bring to the mark with bidistilled water. The shelf life of the solution is no more than six months.
The preparation of an acid solution from a standard titer (fixanal) is carried out in accordance with the instructions for its preparation.
4.3.8 Sodium hydroxide solution, molar concentration 0.2 mol/l
To prepare a 1000 ml solution, 8 g of sodium hydroxide is placed in a beaker, dissolved in bidistilled water, after cooling, the solution is transferred to a 1000 ml volumetric flask and made up to the mark with bidistilled water. The shelf life of the solution in a container made of polymeric material is no more than six months.
4.4 Establishment of the correction factor for the concentration of Trilon B solution
In a 250 ml conical flask, add 10.0 ml of magnesium ion solution (4.3.2), add 90 ml of bidistilled water, 5 ml of buffer solution (4.3.3), 5 to 7 drops of indicator solution (4.3.4.1) or 0.05 to 0.1 g of dry indicator mixture (4.3.4.2) and titrate immediately with the solution. Trilon B (see 4.3.1) until the color changes at the equivalent point from wine-red (red-violet) to blue (with a greenish tint) when using the indicator eriochrome black T, and when using the indicator chrome dark blue acid to blue (blue-violet).
A solution of Trilon B at the beginning of the titration is added quite quickly with constant stirring. Then, when the color of the solution begins to change, the Trilon B solution is added slowly. The equivalent point is reached when the color changes, when the color of the solution stops changing when drops of Trilon B solution are added.
Titration is carried out against the background of a titrated control sample. A slightly overtitrated test sample can be used as a control sample. The result is taken as the arithmetic mean of the results of at least two determinations.
The correction factor for the concentration of Trilon B solution is calculated by the formula
Where is the volume of Trilon B solution used for titration, cm;
10 is the volume of magnesium ion solution (see 4.3.2), see
Note - When preparing solutions according to 4.3, 4.4, it is allowed to use distilled water instead of bidistilled water if the determined hardness value is more than 1 °J.
4.5 Procedure for conducting determinations
4.5.1 Perform two determinations, for which the sample of the analyzed water is divided into two parts.
4.5.2 In a flask with a capacity of 250 ml, place the first part of an aliquot of a 100 ml sample of the water to be analyzed, 5 ml of buffer solution (see 4.3.3), 5 to 7 drops of indicator solution (see 4.3.4.1) or from 0.05 to 0.1 g of the indicator dry mix (see 4.3.4.2) and titrate with a solution of Trilon B (see 4.3.3.3). 3.1) as described in 4.4.
4.5.3 Place the second part of a 100 ml sample aliquot into a 250 ml flask, add 5 ml of buffer solution, 5 to 7 drops of indicator solution or 0.05 to 0.1 g of dry indicator mixture, add Trilon B solution, which is taken 0.5 ml less than that used for the first titration (see 4.5.2), mix quickly and thoroughly and titrate (dotit cut) as described in 4.4.
Notes
1 An indistinct change in color of the indicator at the equivalent point or a change in color to gray indicates the presence of interfering substances. Elimination of interfering influences - according to 4.1. If interfering influences cannot be eliminated, the determination of hardness is carried out by methods of atomic spectrometry (see Section 5).
2 If the consumption of Trilon B solution exceeds 20 cm3 when using a burette with a capacity of 25 cm3 or 9 cm3, or 10 cm3, then the volume of the analyzed sample is reduced by adding bidistilled water to it to a volume of 100 cm3. The sample aliquot is also reduced to eliminate the effect of water color.
3 If the consumption of Trilon B solution is less than 1 ml when using a burette with a capacity of 25 ml or 0.5 ml or 10 ml, then it is recommended to use a solution of Trilon B with a molar concentration of 5 mmol/dm3 or 2.5 mmol/dm3. Trilon B solution according to 4.3.1 is diluted 5 or 10 times.
4.6 Processing the results of the determination
4.6.1
Where is a conversion factor equal to 2, where is the concentration of Trilon B solution, mol / m (mmol / dm), (usually 50);
- correction factor to the concentration of Trilon B solution, calculated by formula (1);
- volume of Trilon B solution used for titration, cm;
4.6.2 The arithmetic mean of the results of two determinations is taken as the measurement result. The acceptability of the results of the determinations is evaluated based on the condition
Where is the repeatability limit (table 1);
and - results of determinations according to 4.5.2 and 4.5.3, °L.
If the discrepancy between the two results exceeds the set value, then the determination of water hardness is repeated. The acceptance test in this case is carried out in accordance with GOST R ISO 5725-6 (section 5).
4.7 Metrological characteristics
The method provides measurement results with metrological characteristics not exceeding the values given in Table 1, with a confidence level of 0.95.
Table 1
| Measurement range of hardness, °J | interval boundaries, | Limit | Reproducibility limit, °J |
| From 0.1 to 0.4 incl. | |||
4.8 Quality control of measurement results
4.8.1 The stability of the measurement results in the laboratory is controlled in accordance with GOST R ISO 5725-6 (section 6) using GSO or GSO solution of the composition of water hardness, which most reflects the value of the hardness of the waters analyzed in the laboratory.
4.8.2 Checking the compatibility of measurement results obtained in two laboratories is carried out in accordance with GOST R ISO 5725-6 (section 5).
If the actual value of the stiffness in the reference sample is unknown, then the results are considered consistent provided
where and are the results of measurements obtained in two laboratories, ° W;
- reproducibility limit for a hardness value of 0.5 (+) (table 1).
If the actual (reference) value of the stiffness in the reference sample is known, then the results are considered consistent provided that
where , - measurement results obtained in two laboratories, ° W;
- reproducibility limit for the stiffness value (table 1);
- actual (reference) value of stiffness in the reference sample, °J.
Note - If in the GRMs used the hardness is expressed in mmol/dm (mol/m), then it is necessary to recalculate into degrees of hardness according to GOST R 52029*.
_______________
* The value of water hardness expressed in mmol/dm is numerically equal to the value expressed in °F.
4.9 Presentation of results
The measurement results are recorded in a protocol (report) in accordance with GOST R ISO / IEC 17025. The protocol indicates the method used in the laboratory according to this standard.
The measurement result can be represented as
where is the value of water hardness, ° W;
- the boundaries of the interval in which the error in determining the hardness of water is with a confidence level of 0.95 (table 1).
5 Methods of atomic spectrometry
5.1 Determination of water hardness by measuring the concentrations of calcium and magnesium ions by flame atomic absorption spectrometry (method B)
The method is based on measuring the resonant absorption of light by free atoms of the chemical elements magnesium and calcium during the passage of light through the atomic vapor of the test sample, which is formed in the flame. To eliminate interfering influences, lanthanum chloride or cesium chloride is added to the sample aliquot.
5.1.1 Sampling - in accordance with section 3.
5.1.2 Measuring instruments, auxiliary equipment, reagents, materials
Scales, laboratory and measuring utensils, auxiliary equipment, materials, bidistilled water, hydrochloric or nitric acid - according to 4.2.
Atomic absorption spectrometer, set up and installed in accordance with the instructions for use, equipped for use with an air-acetylene or nitrous oxide-acetylene flame, a hollow cathode lamp for the determination of calcium and magnesium.
NOTE A nitrous oxide-acetylene flame is recommended if the composition of the samples is complex or unknown, and for samples with a high content of phosphate, sulfate, aluminum ions or silicon.
GRM of the composition of aqueous solutions of magnesium ion and calcium ion with a relative error of certified values of mass concentrations of not more than 1% at a confidence level of 0.95.
Lanthanum chloride heptahydrate, LaCI 7HO or lanthanum oxide LaO, chemically pure, if an air-acetylene flame is used, or cesium chloride CsCI, chemically pure, if a nitrous oxide-acetylene flame is used.
Nitrous oxide.
Air compressed according to GOST 17433.
Acetylene according to GOST 5457.
5.1.3 Preparation of solutions
5.1.3.1 Lanthanum chloride solution, mass concentration of lanthanum 20 g/l
To prepare a 1000 ml solution, slowly and carefully dissolve 24 g of lanthanum oxide in 50 ml of concentrated hydrochloric acid, shaking until the lanthanum oxide is dissolved, transfer the solution to a 1000 ml volumetric flask and make up to the mark with bidistilled water, or dissolve 54 g of lanthanum chloride in 500 to 600 ml of hydrochloric acid solution (see 4.3.7), carried in a volumetric flask with a capacity of 1000 ml and brought to the mark with a solution of hydrochloric acid. The shelf life of the solution is no more than three months.
5.1.3.2 Cesium chloride solution, mass concentration of cesium 20 g/l
To prepare a 1000 ml solution, place 25 g of cesium chloride in a 1000 ml volumetric flask and make up to the mark with hydrochloric acid solution (see 4.3.7). The shelf life of the solution is no more than three months.
5.1.3.3 Calcium-magnesium stock solution
To prepare a stock solution of calcium-magnesium with a mass concentration of calcium 20 mg/dm and magnesium 4 mg/dm, 20.0 cm3 of the composition of an aqueous solution of calcium with a mass concentration of 1 g/dm and 4.0 ml of the GRM of the composition of an aqueous solution of magnesium with a mass concentration of 1 g/dm are added to a volumetric flask with a capacity of 1000 ml with a pipette and brought to the mark with a solution of hydrochloric acid (see 4.3.7). It is allowed to prepare the basic solution of calcium-magnesium with other values of the concentration of calcium and magnesium ions, which most reflect the composition of the analyzed waters. The shelf life of the solution is no more than two months.
5.1.3.4 Calcium and magnesium calibration solutions
To seven 100 ml volumetric flasks add 10 ml of lanthanum chloride solution (5.1.3.1) if an air-acetylene flame is used, or 10 ml of cesium chloride solution (5.1.3.2) if a nitrous oxide-acetylene flame is used; then, the required volume of the basic solution of calcium-magnesium is added to six volumetric flasks (see table 2), it is not added to the seventh flask (blank solution). Dilute all seven flasks to the mark with hydrochloric acid (4.3.7). The shelf life of the solution is no more than one month.
Examples of the resulting concentrations of calcium and magnesium calibration solutions are shown in Table 2.
table 2
5.1.4 Preparing the spectrometer
5.1.4.1 The atomic absorption spectrometer is prepared for operation in accordance with the operation manual (instruction). The values of the analytical wavelengths are 422.7 nm for calcium and 285.2 nm for magnesium.
5.1.4.2 Spectrometer calibration
In accordance with the manual (instruction) for the operation of the spectrometer, calibration solutions are sprayed into the burner flame and the absorption of each element is recorded at the analytical wavelength. In the intervals between calibration solutions, it is recommended to introduce a hydrochloric acid solution. The calibration dependences of the absorption of calcium and magnesium on their content in the calibration solutions are established by the arithmetic mean of the results of three measurements for each calibration solution minus the arithmetic mean of the result of three measurements of the blank solution.
5.1.4.3 Control of the stability of the calibration dependencies is carried out every ten samples, repeating the measurement of one of the calibration solutions. If the measured concentration of this calibration solution differs from the actual one by more than 7%, then the calibration is repeated.
5.1.5 Sample preparation for analysis
In volumetric flasks with a capacity of 100 ml, add 10 ml of a solution of lanthanum chloride if an air-acetylene flame is used, or 10 ml of a solution of cesium chloride if a nitrous oxide-acetylene flame is used, then add an aliquot of the water sample (usually not more than 10 ml) and make up to the mark with a solution of hydrochloric acid (see 4.3.7).
If the measured content of calcium or magnesium in the test sample is higher than the maximum values established during the calibration of the spectrometer, then a reduced volume of the analyzed sample is used for determinations.
Note - When preparing solutions according to 5.1.3-5.1.5, it is allowed to use volumetric flasks of smaller capacity, proportionally reducing the volumes of solutions and aliquots used.
5.1.6 Determination procedure
5.1.6.1 In accordance with the manual (instruction) for the operation of the spectrometer, the analyzed solutions prepared according to 5.1.3.4 are introduced into it, and in the intervals between them, a hydrochloric acid solution (see 4.3.7). Determine the absorption of each element at the analytical wavelength.
5.1.6.2 At the same time, conduct a blank test using the same reagents and in the same quantities as in the preparation of samples according to 5.1.5, replacing the test volume of the test sample with bidistilled water.
Note - When preparing solutions according to 5.1.3-5.1.6, instead of a hydrochloric acid solution, it is allowed to use a solution of nitric acid with a molar concentration of 0.1 mol / dm.
5.1.7 Handling determination results
Based on the calibration dependence (see 5.1.4.2), including using the software of the spectrometer, the mass concentrations of calcium and magnesium in the test solutions and in the blank solution are determined and the content of calcium and magnesium in the sample is calculated, taking into account the dilution of the sample and the value obtained in the experiment with the blank solution.
Water hardness, °F, is calculated by the formula
Where is the mass concentration of the element in the water sample, determined by the calibration dependence, minus the result of the analysis of the blank solution, mg / dm;
- mass concentration of the element, mg/dm, numerically equal to its 1/2 mole;
- dilution factor of the initial water sample during conservation (usually = 1);
- capacity of the flask in which the sample was prepared, according to 5.1.5, cm3;
- volume of water sample taken for analysis, see
5.1.8 Metrological characteristics
The method ensures obtaining the results of measurements of elements (calcium and magnesium) with metrological characteristics not exceeding the values given in Table 3, with a confidence level of 0.95.
Table 3
| Measurement range of element concentration, | The boundaries of the interval in which the measurement error is with a confidence level | Repeatability limit | Reproducibility limit |
| From 1.0 to 50 inclusive | |||
5.1.9 Quality control of the results of determinations - according to 4.8. Instead of the GRM of the composition of water hardness, it is possible to use the GRM of the composition of aqueous solutions of magnesium and calcium ions. The repeatability and reproducibility limits are in accordance with Table 3.
5.1.10 Registration of results - according to 4.9. The value is calculated by the formula
Where - the boundaries of the interval in which the measurement error of the element in the water sample is with a confidence level of 0.95, mg / dm (see table 3);
Note - If it is necessary to calculate the hardness of water, taking into account the content of other alkaline earth elements, the determination of strontium ions is carried out according to GOST 23950, barium - according to GOST R 51309, calculation and presentation of results - according to 5.2.
5.2 Determination of water hardness by measuring the concentrations of alkaline earth ions by inductively coupled plasma atomic emission spectrometry (method B)
5.2.1 Determination of the content of alkaline earth ions (magnesium, calcium, strontium, barium) in a water sample is carried out according to GOST R 51309.
Water hardness, °F, is calculated by the formula
Where is the mass concentration of the element in the water sample, determined according to GOST R 51309, mg/dm;
- mass concentration of the element, mg/dm, numerically equal to 1/2 of its mole.
5.2.2 Quality control of measurement results - according to 4.8. In this case, instead of the GRM of the composition of water hardness, it is possible to use the GRM of the composition of aqueous solutions of magnesium, calcium, barium, strontium ions; the values of the limits of repeatability (convergence) and reproducibility - according to GOST R 51309 (table 4).
5.2.3 Registration of results - according to 4.9. The value is calculated by the formula
Where - the boundaries of the interval in which the relative error in determining the element is with a confidence level of 0.95 according to GOST R 51309 (table 3),%;
- mass concentration of the element in the water sample, determined according to GOST R 51309, mg/dm;
- mass concentration of the element, mg/dm, numerically equal to its 1/2 mole.
5.2.4 If the concentration of strontium and barium ions in the water sample is less than 10% (in total) of the total content of alkaline earth elements, it is allowed not to take into account the content of strontium and barium when calculating water hardness.
ROSSTANDART FA for technical regulation and metrology
NEW NATIONAL STANDARDS: www.protect.gost.ru
FSUE STANDARTINFORM provision of information from the database "Products of Russia": www.gostinfo.ru
FA FOR TECHNICAL REGULATION"Dangerous goods" system: www.sinatra-gost.ru
Determining the hardness of water in the modern world is a prerequisite for ensuring the performance of all equipment working with it. Nevertheless, it cannot be said that such a liquid is really harmful to humans. Everything should be in moderation, because excessively soft water causes no less damage to health than hard water.
The concept of water hardness
You should always start from the very basics, so that there is a complete understanding of the problem. In our case, before proceeding with the determination of water hardness, you first need to understand what it is. According to the results of an examination conducted in 2011 at the Department of Chemistry and Ecology of the Novgorod University named after. Yaroslav the Wise, for natural natural water, hardness is an absolutely normal phenomenon. Until the advent of modern technology, this question was of little interest to anyone; for thousands of years, people calmly used it in the form in which it is. Magnesium and calcium salts dissolved in water give hardness. The very concept of hardness arose as a result of people's feelings, since when water saturated with these salts and other elements interacts with soap, foam practically does not form, making it difficult to wash or wash.
Types of stiffness
Before understanding what kind of water to drink, one should take into account the fact that hardness is not a homogeneous quantity. There are at least two:
- Temporary.
- Constant.
These types depend on the type of dissolved salts, which are always present in any hard water together, making up the total hardness. However, they can and should be separated. Temporary hardness directly depends on the presence of bicarbonate and hydrocarbonate anions. Their main feature is decomposition during boiling. As a result of the decomposition, water itself, carbon dioxide and calcium carbonate are obtained directly, which is practically insoluble. It turns out that you can get rid of temporary stiffness without any problems by simply raising the water temperature to +100 degrees. An example is any kettle. After prolonged use, a precipitate can be found inside, which is the result of the decomposition process described above. Everything that does not decompose in this way refers to permanent hardness, which is almost impossible to get rid of without special treatment.

Why you need to know the hardness of water
This is necessary in order to understand what kind of water you can drink safely, and also so that any equipment that interacts with water does not fail. For a person, excessively hard water is harmful. But even if this parameter is at an acceptable level for our body, the equipment will still not suit it. Aquariums, coffee, washing and dishwashers, kettles, multicookers and many other equipment options require water of a strictly defined hardness. Usually filters such as Geyser-3 help to cope with this, but often such a measure can even be considered unnecessary. Before spending money on them, it is recommended that you first test for water hardness, because it is quite possible that this indicator is already at a normal level.
Harm of hard and soft water
As mentioned above, first of all, it is not a certain type of water that causes damage to a person, but a complete lack of balance in the body.
Effects of hard water:
- Poor dissolution of foods (associated with Ca 2 + and Mg 2 + cations).
- Coffee, tea and any other similar drinks are brewed very poorly.
- With prolonged use, relaxation of the stomach is possible.
- Hard water can cause kidney stones to form.
- Saturates the body with the elements it needs.
- Improves the condition of the teeth, reduces the likelihood of caries.
- Hard water is the cause of the breakdown of most types of equipment.
Effects of soft water:
- Removes toxins, but along the way washes away useful elements (potassium, magnesium and calcium). As a result, the bones become more fragile. It also does not have the best effect on the cardiovascular system.
- Negatively affects the pituitary-adrenal system.
- It has a bad effect on the water-salt balance of the body.
Thus, the determination of water hardness should not be done in order to get rid of it, but in order to minimize the negative impact and bring the use of such a liquid to the balance required by the body.

Sampling rules according to GOST
According to GOST, drinking water must be tested for hardness strictly in the laboratory, by means of titrimetric analysis. To do this, you first need to take samples, the volume of which must be at least 400 cubic centimeters (0.4 liters). As a container in which storage will be carried out, any container can be used if it is made of glass or polymeric material. It is very important to carry out the analysis no later than 24 hours after sampling. In special cases, when it is necessary to increase this period, the liquid is acidified by adding hydrochloric acid. In this state, it can be stored for about 1 month.
Titrimetric (laboratory) analysis
Among all water hardness, this option is deservedly considered the most reliable and complex. It is based on the process of formation of Trilon compounds together with alkaline earth ions. The minimum hardness index that can be determined using this method is 0.1 o F (7-10 o F is considered the norm). Ordinary tap water can be used as a sample. The best way out in a situation with suspected increased stiffness is to immediately visit the appropriate laboratory, since no home methods can give accurate data. But about them - below.
It makes no sense to fully describe the whole process, since it is impossible to reproduce it on your own, without the necessary skills and chemical elements and equipment. Nevertheless, there are several basic principles of reaction that remain in any situation and are inherent in absolutely all options:
- There must always be a way to fix the equivalence of the reaction, which is the basis for determining the stiffness.
- The analysis is carried out very quickly.
- The requirement of stoichiometric process must be met. Simply put, this means that no side products should form during the reaction.
- Once a reaction has begun, it cannot be reversed or stopped.

test strips
To determine the hardness of water at home, you can use special devices, which will not be difficult to buy (they are not prohibited and publicly available). They look like standard test strips. To use, it is enough to immerse one of them in the water that needs to be checked for the period of time specified in the instructions. As a result, the product will change its color. When using such strips to determine the hardness of water, the main problem is to determine what exactly the hardness indicator is. To do this, you need to compare the color on the strip and the examples with the description on the package. Unfortunately, it is far from always possible to immediately understand what exactly the device shows, and even in a clearer situation, the accuracy of the data leaves much to be desired. In general, such test strips are only suitable for a general understanding of how hard or soft water is.

home analysis
You can also check tap water for hardness using improvised means. True, this is more entertaining than really an option for testing fluid readings.
You need to take:
- A jar with a capacity of 1 liter (or any other similar capacity).
- A glass in the form of a cylinder.
- Any scales (it is most convenient to use electronic).
- Ruler.
- Laundry soap (72% or 60%).
- distilled water.
To check, you need to take 1 gram of soap, grind it and place it in a glass. After that, distilled water should be heated, but not brought to a boil. It should be poured into the glass in which the soap already lies. As a result, it must dissolve in water. The next step is to pour even more water. After that, pour into a jar of ordinary tap water and slowly pour the soapy liquid from the glass and mix (slowly). If foam is formed, then this is an indicator of stiffness. Unfortunately, it is almost impossible to say more or less clearly what exactly its level is using this method.

TDS analysis
Another option for determining the hardness of drinking water is to use a special device - a TDS meter. In principle, it is intended to determine what is affected both directly by salts (creating hardness) and many other elements, which does not give the desired level of accuracy. Moreover, an ordinary person who does not know how to read them will not understand the readings of the device and is likely to get confused. Let's try to simplify the task. The vast majority of such devices use some ppm as units of measurement. We also use other options based on the equivalent of a milligram per liter of liquid. On average, 1 our unit (mg-eq / l) is equal to 50.05 foreign ppm. According to the rules, the concentration of salts (i.e. hardness) should be no more than 350 ppm or 7 mg-eq / l. These numbers are worth looking into. If the device is domestic, everything is greatly facilitated. Worst of all, when such a device is made somewhere in China or another similar country, which uses its own units of measurement. Then you will have to independently look for their equivalent and translate it into the testimony we are used to.
AKMS-1
Of the other devices capable of determining the hardness of water, the unique AKMS-1 device should be noted separately. This is a fairly large stationary unit, similar in size to the Geyser-3 filters. Just like that, at home, it is not possible to check the liquid with it. That is why such devices are used primarily in production, where water hardness can affect the operation of expensive equipment or cause other similar harm. Unlike all other analogues, AKMS-1 really quickly and accurately shows the current level of rigidity, allowing the operator to respond in a timely manner. With the help of this device, you can either let water go directly to the working units, if it does not pose a threat to them, or pre-filter it. This, of course, will result in additional costs, but it will help save on the repair of equipment, which will cost much more.

Results
Given all of the above and the requirements of GOST, drinking water should be regularly checked for hardness levels. Nevertheless, it is not worth taking radical measures to soften it, since both conditions are harmful - too hard and too soft. Only in the situation when the indicators are really higher or lower, it is worth taking some action. By the way, if hardness is regularly fought, then it is almost unheard of about too soft water, but you also need to pay no less attention to this.
It is believed that hard water is unpleasant in taste and is easily recognizable by the dense layer of scale on the inside of the kettle and other heating appliances. Meanwhile, in laboratory conditions, the concentration of magnesium and calcium salts is determined using a whole set of measuring instruments and auxiliary reagents.
Determination of water hardness is one of the main stages of water treatment. In order to measure the concentration of calcium and magnesium salts, laboratory chemists use only instruments and indicators approved by GOST requirements. To begin with: what is water hardness and how to detect it at home.
To date, the following methods for determining stiffness are considered the most reliable:
Complexometric method for detecting total and temporary hardness,
Atomic spectrometry method
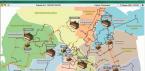
Bymap of acidity and hardness of water in RussiaYou can estimate approximate indicators for your region.
How to determine the total hardness of water according to GOST?
To determine the total hardness of water in the laboratory, a complexometric method is used, based on the formation of complex compounds of the analyzed ions with organic reagents (complexons) (1). Before starting work, the sample is diluted with an alcohol solution of the eriochrome black T indicator or a dry mixture of sodium chloride and calcium. Trilon B is added dropwise to the wine-red solution. The value of the total hardness is calculated by the formula:
Jo=Nx*Vx*1000/V1
(N-normality of Trilon B solution, V-volume of Trilon B solution, V1-sample volume).
For reference: Titration (titrimetric analysis) is a method of quantitative calculation of the content of a substance that reacts with a reagent of known concentration.
How to check the temporary hardness of water according to GOST?
With the help of titration and the colorimetric method, you can find out not only the value of the total, but also the temporary hardness. To do this, the test samples are combined with an indicator (methyl orange), after which the reference sample is moved to a white background, and the second test tube is titrated with hydrochloric acid until an orange-red tint appears. Calculating the required amount of "hodgepodge", determine the temporary hardness of the water.
Formula:
Hvr \u003d N HCl * V HCL * 1000 / V1
(N-normality of hydrochloric acid solution, V-volume of hydrochloric acid solution, V1-sample volume)
Reaction equation:
CaCO 3 + 2HCl \u003d CaCl 2 + CO 2 + H 2 O
Ca(HCO 3) 2 + 2HCl \u003d CaCl 2 + 2H 2 O + 2CO 2
How to determine the hardness of water with high accuracy?
Titration is one of the most common and simple methods for determining the concentration of calcium and magnesium ions. The disadvantages of traditional methods include low accuracy.
The service personnel of high-precision instruments knows how to check the hardness of water with minimal errors. A striking example of one of the most reliable tools for determining the concentration of calcium and magnesium ions is AKMS-1. According to the results of comparison of the electrode potential difference with the reference values, the device automatically displays the analysis results on the display.
The method of atomic spectrometry is based on the resonant absorption of light by the atoms of the studied chemical elements. The advantage of this method is high accuracy. The disadvantage of atomic spectrometry is the high cost of the required instruments.
How to find out the hardness using household appliances?
To determine the hardness of water, you can use the devices and tools used in the aquarium hobby. TDS meters take readings with an accuracy of 2%.
The principle of operation of such a device is based on a direct relationship between the electrical conductivity and the amount of dissolved calcium and magnesium salts.
At factories and enterprises, as well as in the laboratory conditions of treatment facilities, the most accurate results are considered to be the results of several experiments that differ in the method of calculating the concentration of "target" components or pollutant particles.
Sources used.
Federal Agency for Education
State educational institution
Department of Life Safety and Chemistry
complexometric method
in the disciplines "Ecology",
"Industrial Ecology"
for students of all courses,
Rostov-on-Don
Compiled by:
candidate of technical sciences, associate professor L.M. Raspopov
Candidate of Chemical Sciences, Associate Professor R.P. Boyko
associate professor P.V. tunic
UDC 504.006.331.45
Determination of the hardness of drinking water by the complexometric method: Method. instructions for laboratory work in the disciplines "Ecology", "Biology with the basics of ecology", "Industrial ecology" / RGASCM; Ed. prof. V.L. Gaponova; Rostov n / D., 2008. - 5 p.
The guidelines set out the hygienic and technical and technological aspects of the hardness of drinking water, the methodology for its determination and sanitary and hygienic assessment.
Designed for students of all courses, specialties and forms of education.
Published by decision of the editorial and publishing council
Rostov-on-Don State Academy
agricultural engineering
Reviewer candidate of technical sciences, associate professor
IN AND. Garshin
Scientific editor candidate of technical sciences, associate professor
L.H. Badalyan
State educational institution
higher professional education
Rostov-on-Don State Academy
agricultural engineering, 2008
Safety instructions
When performing laboratory work “Determining the hardness of drinking water by the complexometric method”, students must observe the following rules:
1. Handle glassware or chinaware and equipment with care.
2. Be careful with acid solutions! Avoid contact of acids with clothing, skin, eyes and surrounding objects.
3. Carry out the work carefully and accurately in accordance with the instructions.
4. After finishing work, wash all the dishes, clean the equipment and the workplace.
5. With all unclear questions, contact the teacher.
1. The purpose of the work
1.1. To consolidate theoretical knowledge about the hygienic and techno-technological significance of water hardness.
1.2. Master the skills of determining the sanitary and hygienic examination of water quality by hardness.
2. General provisions
Hardness of water is a property of water due to the presence of Ca 2+ and Mg 2+ ions in it. The use of hard water leads to the deposition of solid sediment (scale) on the walls of steam boilers, heat exchangers, makes it difficult to cook food, washing. As a result of experimental and clinical and medical studies, an adverse effect on the body has been established. rigidity water, caused by the total content of calcium and magnesium salts in it. High stiffness may play an etiological (causal) role in the development of human urolithiasis. Urologists even single out the so-called “stone” zones - territories where urolithiasis can be considered an endemic disease (endemia is the permanent existence of a certain disease in any territory).
Distinguish carbonate hardness associated with the presence of a bicarbonate ion in water HCO 3 , And non-carbonate hardness due to the presence of chlorides and sulfates in water.
Carbonate hardness can be temporary or permanent. Temporary hardness of water is eliminated after boiling (at least 10 minutes) as a result of the decomposition of bicarbonates and the formation of insoluble carbonates:
Ca(HCO 3 ) 2 =CaCO 3 + H 2 O+CO 2
Mg(HCO 3 ) 2 =MgCO 3 + H 2 O+CO 2
The permanent hardness remaining in water after 10 minutes of boiling is made up of non-carbonate and partially carbonate hardness. Permanent hardness can be eliminated by softening the water by adding slaked lime, soda, using cations, etc.
The sum of carbonate and non-carbonate hardness is generalrigidity .
Water hardness is measured in millimoles per liter (mmol/l) (1 mmol/l of hardness corresponds to 56 mg of calcium oxide or an equivalent amount of magnesium oxide). Water with a hardness of up to 1.75 mmol / l is considered soft; from 1.75 to 3.5 mmol / l - medium hardness; from 3.5 to 17 mmol / l - hard and over 17 mmol / l - very hard.
The determination of the total hardness of water is carried out according to GOST 4151-72 using the complexometric method.
3. Material support
0.1 N hydrochloric acid solution (n. - the normality of the solution).
2. Methyl orange (indicator).
Ammonia buffer solution.
Erichrome black (indicator).
0.1n. Trilon B solution (disodium salt of ethylenediaminetetra-acetic acid).
Flask with a capacity of 100 ml.
Flask conical with a capacity of 250 ml.
Pipettes per (100 ml, 5 ml).
4. Order of work
4.1 Determine the temporary hardness of the water.
To do this, pour 100 ml of the test water into the flask, add 2 drops of methyl orange and titrate with 0.1 N. hydrochloric acid solution, stirring vigorously until the yellow color changes to slightly pink. Each milliliter is 0.1 N. hydrochloric acid solution corresponds to 1 mmol of hardness. Calculate the temporary hardness of the investigated water (H in) according to the formula:
H V =a T 1000/ V,
where V is the volume of the water sample, ml;
a - consumption for titration 0.1 N. HCl solution, ml;
T is the titer of nitric acid, mmol/ml;
Make an assessment of the degree of temporary hardness of water.
4.2 Determine the total hardness of water by the complexometric method. The method is based on the ability of Trilon B to bind Ca 2+ and Mg 2+ ions into strong complexes. At the moment when the Ca 2+ and Mg 2+ ions in the water are bound into a complex, the erychrome black indicator added in advance will change its initial color from red to blue with a greenish tint.
Pour 100 ml of analyzed water into a conical flask, add 5 ml of ammonia buffer solution and 6 drops of an indicator (erychrome black), then titrate with 0.1 N. Trilon B solution, stirring vigorously until the color of the water changes to bluish-green. Each milliliter is 0.1 N. Trilon B solution corresponds to 0.1 mmol of hardness.
Calculate the total hardness of the analyzed water (H) using the formula:
H V =a T 1000/ V,
where a is the consumption for titration of 0.1 N. Trilon B solution, ml;
T - titer of Trilon B, mmol/ml;
1000 - coefficient for conversion to 1 liter of water.
Make a hygienic assessment of the total hardness of water, taking into account the sanitary standard - no more than 7 mmol / ml.
The name of the work, its purpose.
Formulation of water hardness.
Hygienic value of water hardness.
Types of water hardness and methods for its elimination.
Calculations of temporary and general stiffness.
Table with research results.
Conclusion on the hygienic compliance of drinking water with the standards for total hardness.
Study protocol
6. Security questions
6.1 What is water hardness and what does it depend on?
6.2 Which water is called soft, medium hard and hard?
6.3 What is the impact of water hardness on human health?
6.4 What is the technical and technological aspect of water hardness?
6.5 What are the types of stiffness?
6.6 What are the methods for removing stiffness?
6.7 What is the sanitary and hygienic norm of total hardness in drinking water in accordance with SanPiN 2.1.4.559-96?
LITERATURE
1. Ecology and nature management / Ed. E.A. Aristumov. – M.: Ed. House "Dashkov and K", 1999.
2. Gurova A.I., Gorlova O.E. Workshop on general hygiene. - M .: University of Friendship of Peoples, 1991.
3. Rabinovich R.D. Hygiene. – M.: Medicine, 1982.
4. SanPin 2.1.4.559–96. Sanitary rules and regulations. Drinking water. Hygienic requirements for water quality. – M.: Goskomsanepidnadzor of Russia, 1996.
Educational and methodical edition
Compiled by:
Raspopova Ludmila Maksimovna
Boyko Raisa Petrovna
tunic Petr Vladimirovich
determination of the hardness of drinking water
complexometric method
Guidelines for laboratory work
in the disciplines "Ecology",
"Biology with the basics of ecology",
"Industrial Ecology"
for students of all courses,
specialties and forms of education
Editor T. Yu. Karasevich
Persons LR No. 020824 dated 10/20/98
Signed for printing _________ Paper size 60x84/16
Offset paper Volume 0.5 arb. p.l., 0.6 ac.-ed. l.
Order No. ____ Circulation 50 copies.
____________________________________________________
Editorial and publishing department of RGASKhM GOU
Rostov-on-Don, st. Soviet countries, 1
Printed at the copying and duplicating bureau of the RGASKM GOU
All documents presented in the catalog are not their official publication and are intended for informational purposes only. Electronic copies of these documents can be distributed without any restrictions. You can post information from this site on any other site.
Foreword
The goals and principles of standardization in the Russian Federation are established by the Federal Law of December 27, 2002 No. 184-FZ "On Technical Regulation", and the rules for the application of national standards of the Russian Federation - GOST R 1.0-2004 "Standardization in the Russian Federation. Basic Provisions»
About the standard
1 DEVELOPED AND INTRODUCED by the Technical Committee for Standardization TK 343 "Water Quality" (SUE Center for Research and Water Control, FSUE VSEGINGEO, FSUE VNIIstandart, OOO Protector)
2 APPROVED AND INTRODUCED BY Order No. 317-st of December 20, 2005 of the Federal Agency for Technical Regulation and Metrology
3 This standard takes into account the main normative provisions of the following international ISO standards:
ISO 6059-1984 “Water quality. Determination of the total content of calcium and magnesium. Titrimetric method using EDTA" ( ISO 6059-1984 "Water quality - Determination of the sum of calcium and magnesium - EDTA titrimetric method" » (section 4 of this standard);
ISO 7980-1986 “Water quality. Determination of calcium and magnesium. Atomic absorption spectrometric method" ( ISO 7980-1986 Water quality - Determination of calcium and magnesium - Atomic absorption spectrometric method "") (clause 5.1 of this standard);
ISO 11885-1996 “Water quality. Determination of 33 elements by atomic emission with inductively coupled plasma” ( ISO 11885-1996 "Water quality - Determination of 33 elements by inductively coupled plasma atomic emission spectroscopy" "") (clause 5.2 of this standard)
4 INTRODUCED FOR THE FIRST TIME
Information about changes to this standard is published in the annually published information index "National Standards", and the text of changes and amendments - in the monthly published information indexes "National Standards". In case of revision (replacement) or cancellation of this standard, a corresponding notice will be published in the monthly published information index "National Standards". Relevant information, notification and texts are also posted in the public information system - on the official website of the national body of the Russian Federation for standardization on the Internet
Introduction
Water hardness is one of the main indicators characterizing the use of water in various industries.
Water hardness is a set of properties determined by the content of alkaline earth elements in it, mainly calcium and magnesium ions.
Depending on the pH and alkalinity of the water, hardness above 10°H can cause sludge in the water distribution system and scale when heated. Water with a hardness of less than 5°H can be corrosive to water pipes. The hardness of water can also affect the applicability for human consumption in terms of its taste properties.
In the complexometric (titrimetric) determination of hardness, aluminum, cadmium, lead, iron, cobalt, copper, manganese, tin and zinc ions affect the establishment of the equivalent point and interfere with the determination. Orthophosphate and carbonate ions can precipitate calcium under titration conditions. Certain organic substances may also interfere with the determination. If the interfering influence cannot be eliminated, it is recommended to determine the hardness using atomic spectrometry methods.
This standard provides for the use of various methods for determining water hardness, taking into account bringing the quantitative characteristics of water hardness (hardness units) in accordance with GOST R 52029.
Introduction date - 2007-01-01
1 area of use
This standard applies to drinking and natural waters, including drinking water sources, and establishes the following methods for determining water hardness:
Complexometric method (method A);
Methods of atomic spectrometry (methods B and C).
Method B according to 5.1 is used to determine the mass concentration of calcium and magnesium ions.
Method B of 5.2 is an arbiter of other stiffness methods.
2 Normative references
This standard uses normative references to the following standards:
In a conical flask with a capacity of 250 cm 3, add 10.0 cm 3 of a solution of magnesium ions (see), add 90 cm 3 of bidistilled water, 5 cm 3 of a buffer solution (see), from 5 to 7 drops of an indicator solution (see) or from 0.05 to 0.1 g of a dry mixture of the indicator (see) and immediately titrate with a solution of Trilon B (see) until the color changes in an equivalent point from wine-red (red-violet) to blue (with a greenish tint) when using the indicator eriochrome black T, and when using the indicator chrome dark blue acidic to blue (blue-violet).
A solution of Trilon B at the beginning of the titration is added quite quickly with constant stirring. Then, when the color of the solution begins to change, the Trilon B solution is added slowly. The equivalent point is reached when the color changes, when the color of the solution stops changing when drops of Trilon B solution are added.
Titration is carried out against the background of a titrated control sample. A slightly overtitrated test sample can be used as a control sample. The result is taken as the arithmetic mean of the results of at least two determinations.
Correction factor TO to the concentration of Trilon B solution is calculated by the formula
,(1)
Where V- volume of Trilon B solution used for titration, cm3;
10 - the volume of a solution of magnesium ions (see), cm 3.
Note - When preparing solutions according to 4.3, 4.4, it is allowed to use distilled water instead of bidistilled water, if the determined hardness value is more than 1°L.
If the actual (reference) value of the stiffness in the reference sample is known, then the results are considered consistent provided that
|AND L1- AND L 2 | ≤ Rµ (5)
Where AND L1, AND L2 - measurement results obtained in two laboratories, ° W;
R µ - reproducibility limit for the stiffness value µ ();
µ is the actual (reference) value of stiffness in the reference sample, °F.
Note - If in the GRMs used, the hardness is expressed in mmol / dm 3 (mol / m 3), then it is necessary to recalculate into degrees of hardness according toGOST R 52029 1)
1) The value of water hardness, expressed in mmol / dm 3, is numerically equal to the value expressed in ° F.
In accordance with the manual (instruction) for the operation of the spectrometer, calibration solutions are sprayed into the burner flame and the absorption of each element is recorded at the analytical wavelength. In the intervals between calibration solutions, it is recommended to introduce a hydrochloric acid solution. The calibration dependences of the absorption of calcium and magnesium on their content in the calibration solutions are established by the arithmetic mean of the results of three measurements for each calibration solution minus the arithmetic mean of the result of three measurements of the blank solution.
5.1.4.3 Control of the stability of the calibration dependencies is carried out every ten samples, repeating the measurement of one of the calibration solutions. If the measured concentration of this calibration solution differs from the actual one by more than 7%, then the calibration is repeated.
5.1.5 Sample preparation for analysis
In volumetric flasks with a capacity of 100 cm 3, add 10 cm 3 of a solution of lanthanum chloride, if an air-acetylene flame is used, or 10 cm 3 of a solution of cesium chloride, if a nitrous oxide-acetylene flame is used, then add an aliquot of the water sample (usually not more than 10 cm 3) and bring to the mark with a solution of hydrochloric acid (see).
If the measured content of calcium or magnesium in the test sample is higher than the maximum values established during the calibration of the spectrometer, then a reduced volume of the analyzed sample is used for determinations.
Note - When preparing solutions according to - 5.1.5, it is allowed to use volumetric flasks of smaller capacity, proportionally reducing the volumes of solutions and aliquots used.
5.1.6 Determination procedure
5.1.6.1 In accordance with the manual (instruction) for the operation of the spectrometer, the analyzed solutions are introduced into it, prepared according to , and in the intervals between them, a solution of hydrochloric acid (see ). Determine the absorption of each element at the analytical wavelength.
5.1.6.2 At the same time, conduct a blank test using the same reagents and in the same quantities as in the preparation of samples according to 5.1.5, replacing the test volume of the test sample with bidistilled water.
Note - When preparing solutions according to - 5.1.6, instead of a hydrochloric acid solution, it is allowed to use a solution of nitric acid with a molar concentration of 0.1 mol / dm 3.
5.1.7 Handling determination results
Based on the calibration dependence (see ), including using the software of the spectrometer, the mass concentrations of calcium and magnesium in the studied solutions and in the blank solution are determined and the content of calcium and magnesium in the sample is calculated, taking into account the dilution of the sample and the value obtained in the experiment with a blank solution.
Hardness of water AND, ° W, calculated by the formula
AND = ∑(WITH i / WITH i e )· F· V To / V p ,(7)
Where WITH i , - mass concentration of the element in the water sample, determined by the calibration dependence, minus the result of the analysis of the blank solution, mg/dm 3 ;
WITH i e - mass concentration of the element, mg / dm 3, numerically equal to its 1/2 mole;
F - dilution factor of the initial water sample during conservation (usuallyF = 1);
V To - the capacity of the flask in which the sample was prepared, by , cm 3;
V P - volume of water sample taken for analysis, cm 3 .
5.1.8 Metrological characteristics
The method ensures obtaining the results of measurements of elements (calcium and magnesium) with metrological characteristics not exceeding the values given in Table 3, with a confidence level R= 0,95.
Table 3
5.1.9 Quality control of the results of determinations - according to. Instead of the GRM of the composition of water hardness, it is possible to use the GRM of the composition of aqueous solutions of magnesium and calcium ions. The repeatability and reproducibility limits are in accordance with Table 3.
5.1.10 Registration of results - according to. Meaning Δ calculated according to the formula
![]() ,(8)
,(8)
Where Δ e - the boundaries of the interval in which the measurement error of the element in the water sample is with a confidence probability R\u003d 0.95, mg / dm 3 (see table 3);
WITH i e - mass concentration of the element, mg / dm 3, numerically equal to its 1/2 mole.
Note - If it is necessary to calculate the hardness of water, taking into account the content of other alkaline earth elements, the determination of strontium ions is carried out according to GOST 23950, barium - according to GOST R 51309 , calculation and presentation of results - according to 5.2.
5.2 Determination of water hardness by measuring the concentrations of alkaline earth ions by inductively coupled plasma atomic emission spectrometry (method B)
5.2.1 Determination of the content of alkaline earth ions (magnesium, calcium, strontium, barium) in a water sample is carried out according to GOST R 51309.
Hardness of water AND, ° W, calculated by the formula
AND= ∑(С i /С i e ),(9)
Where WITH i
WITH i e - mass concentration of the element, mg / dm 3, numerically equal to 1/2 of its mole.
5.2.2 Quality control of measurement results - according to . In this case, instead of the GRM of the composition of water hardness, it is possible to use the GRM of the composition of aqueous solutions of magnesium, calcium, barium, strontium ions; the values of the limits of repeatability (convergence) and reproducibility - according to GOST R 51309 (table 4).
5.2.3 Registration of results - according to. Meaning Δ calculated according to the formula
![]() ,(10)
,(10)
where δ - the boundaries of the interval in which the relative error in determining the element is with a confidence probability R\u003d 0.95 according to GOST R 51309 (table 3),%;
WITH i - mass concentration of the element in the water sample, determined according to GOST R 51309, mg / dm 3;
C i e - mass concentration of the element, mg / dm 3, numerically equal to its 1/2 mole.
5.2.4 If the concentration of strontium and barium ions in the water sample is less than 10% (in total) of the total content of alkaline earth elements, it is allowed not to take into account the content of strontium and barium when calculating water hardness.